Which of the Following Is Not an Empirical Formula
The molecular formula of a compound can be the same as its empirical formulab. An oxide of chromium is found to have the following composition.

Solved Question 2 Which Of These Is Not An Empirical Chegg Com
Learn about our Editorial Process.
. Calculate the following to four significant figures. Experts are tested by Chegg as specialists in their subject area. The percent composition of a compound was found to be 635 silver 82.
The Formula of Autonomy takes something important from both the Formula for the Universal Law of Nature and the Formula of Humanity. The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound but not the actual numbers of atoms found in the molecule. Which of the following is NOT true about empirical and molecular formulas.
A periodic table will be required to complete this practice test. Option A - The molecular formula can be a simple whole number multiple of the empirical formula. Which one of the following is not an empirical.
Count the number of moles of each type of atom that is present. The molecular formula of a compound can be some whole-number multiple of its empirical formulac. The lowest whole-number ratio of the elements in a compound is called the empirical formula.
See all questions asked by the same visitor. It is molecular formula of ethane. The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound.
MoO2Cl2 BeCr2O7 C2N2H8 C3H8O. By using the expression Molecular formula n empirical formula. What is its molecular formula.
Which of the following is an empirical formula P4O10 C2H8N2 C3H6O2 H2O2. 50 can be entered as 50 or 50. Which of the following is NOT an empirical formula.
This problem has been solved. 5 O 3 6. This 10-question practice test deals with finding empirical formulas of chemical compounds.
4005 S and 5995 O. The empirical formula for trichloroisocyanuric acid the active ingredient in many household bleaches is OCNCI. To calculate the empirical formula enter the composition eg.
Enter an optional molar mass to find the molecular formula. Select the correct answer below. The percent composition of.
But the measured molecular mass for Boron atom is given as 2766u. Empirical formula of C_2 H_6 is CH_3. OCH ОСН OCH CH2.
We review their content and use your feedback to keep the quality high. What mass of water is formed if 080 g of methane reacts with 32 g of oxygen to produce 22 g of carbon dioxide. Molecular formulas are derived by comparing the compounds molecular or molar mass to its empirical formula mass.
N molecular formulaempirical formula. 2 hours agoThe Formula of Autonomy and the Kingdom of Ends. Which of the following molecules does NOT have the empirical formula CH.
Updated 1252016 122721 PM. Since the subscripts of the elements in C_6H_12O_6 can be divided by 6 to get the simplest whole number ratio of elements it is not an empirical formula. 78 Which one of the following isnotan empirical formula.
Which of the following is not an empirical formula. For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. C40 H667 O533 of the compound.
The Formula for the Universal Law of Nature involves thinking about your maxim as if it were an objective law while the Formula of Humanity is more subjective and. 684 Cr and 316 O. What is the empirical formula of a compound that is 660 calcium and 340 phosphorus A.
Molecular formula 2 BH 3 B 2 H 6. C 5 4. An organic compound on analysis gave the following results.
C H O 120 g C H O. The molar mass of. C_2 H_6 is not an empirical formula.
This is because we can divide each number in C 6. Updated on July 03 2019. The ratios are denoted by subscripts next to the element symbols.
310 79 Methane and oxygen react to form carbon dioxide and water. Dividing each subscript by 6 gives the empirical formula CH_2O. Determine this compounds empirical formula.
The steps for determining a compounds empirical formula are as follows. All of the following are empirical formulas EXCEPT_____ C6H5Cl N2O4 Sn3PO44 Na2SO4 14 g N 80 g NH4NO3 X 100. 4 H 9.
Which of the following is not an empirical formula. As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. A CHOB CH2O C C2H4O D C2H4O2 Answer.
Find the empirical formula for these elements. Option A - The molecular formula can be a simple whole number multiple of the empirical formula. Calculate the mass of each element in grams.
The empirical formula of the compound is The empirical formula of the compound is Hard. Percentages can be entered as decimals or percentages ie. Divide the number of moles of each element from the smallest number of moles found in the previous step.
CH N 90 g C H N. Which of the following is NOT an empirical formula. Who are the experts.
This program determines both empirical and molecular formulas. An empirical formula represents the simplest whole number ratio of elements in a compound. You are given the following percentages.
C2N2H8-is NOT an empirical formula Expert answered. A compound has the empirical formula C2H3O2 and a molecular mass of 177. Putting value of n 2 in the empirical formula we get molecular formula as.

Answered Which Of The Following Is Not An Bartleby

Solved 3 Which One Of The Following Is Definitely Not An Empirical Formula 2 Points 10 Cuhuo Cho Chuoz Crzor Chhj

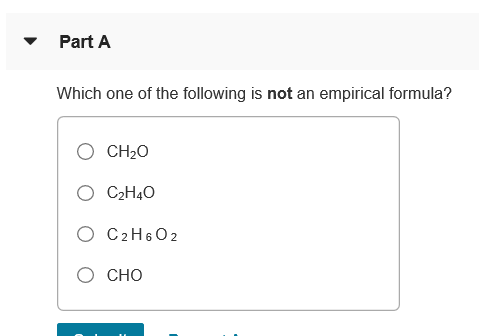
Solved Part A Which One Of The Following Is Not An Chegg Com
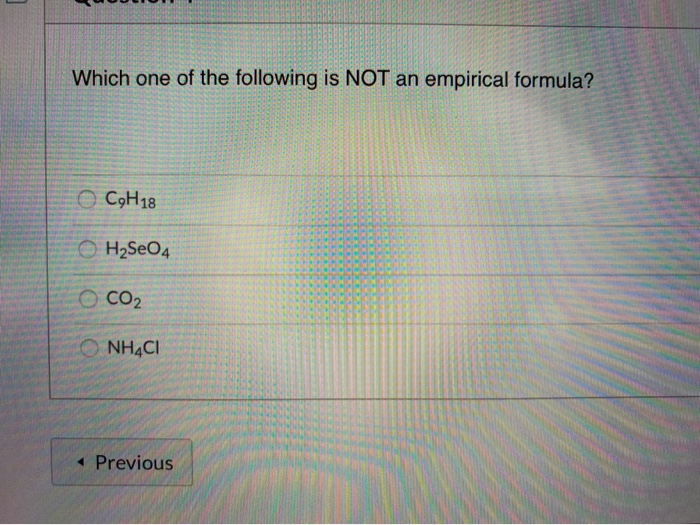
Solved Which One Of The Following Is Not An Empirical Chegg Com
No comments for "Which of the Following Is Not an Empirical Formula"
Post a Comment